Infected droplets can stay longer on smartphone screens than on other glass surfaces : IIT-H study
An interdisciplinary research group comprising faculty at the Indian Institute of Technology Hyderabad predicted the life of SARS-CoV-2 droplets on surfaces in different environmental conditions.
By Newsmeter Network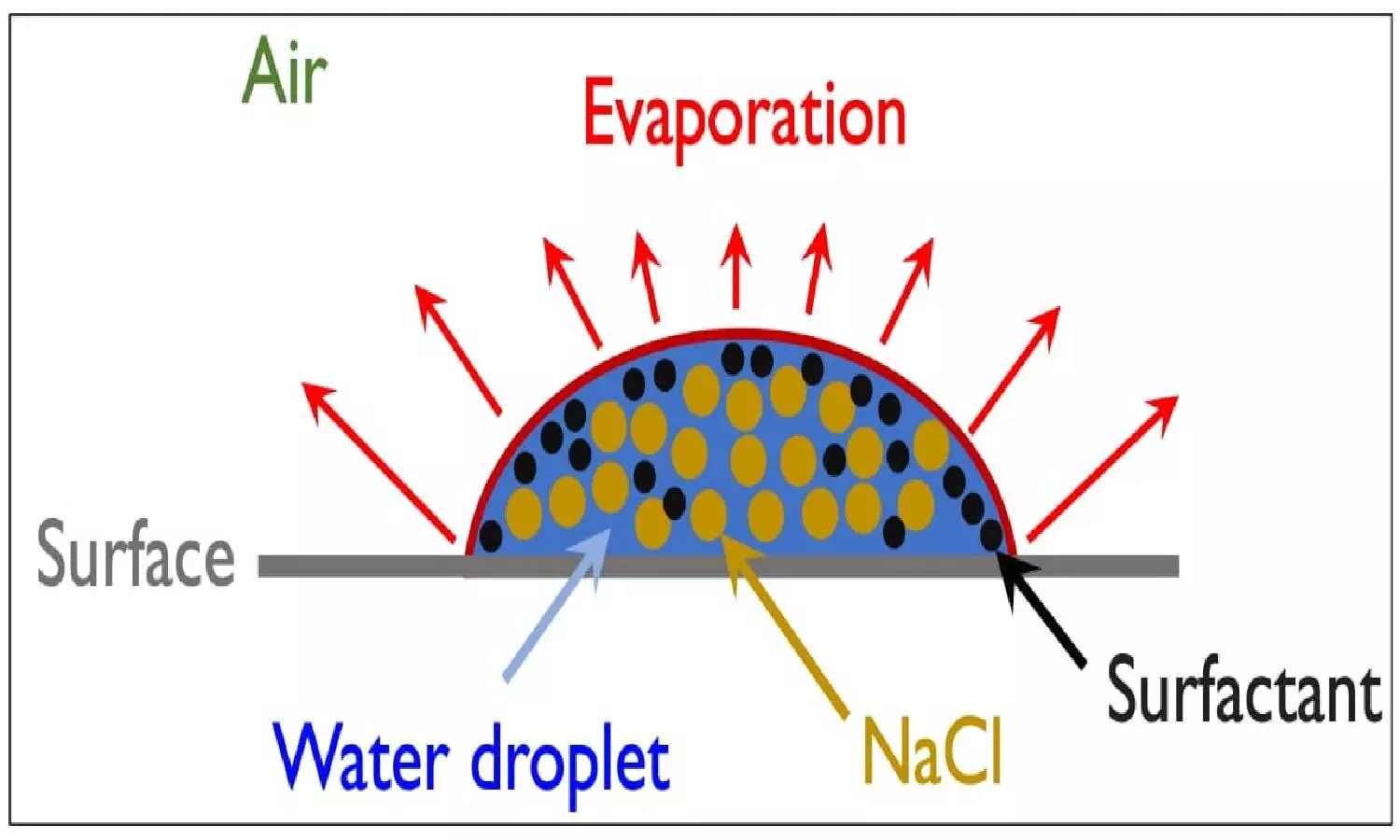
Hyderabad: An interdisciplinary research group comprising faculty at the Indian Institute of Technology Hyderabad predicted the life of SARS-CoV-2 droplets on surfaces in different environmental conditions. The drying time of droplets on various surfaces differs due to varying nature of conditions such as ambient temperature, relative humidity, and volume and composition of droplets.
Under the same environmental conditions, the drying time for droplets on a smartphone screen is three times longer than that on a normal glass surface.
Respiratory infections such as the SARS-CoV-2 virus spreads primarily by respiratory droplets (size > 10μm) of saliva or discharge from the nose of an infected person during coughing/sneezing. Fluid dynamics play an important role in the study of the life of these droplets migrating in the air or after they get deposited on surfaces. The study also showed the factors that significantly delay the evaporation of respiratory droplets in comparison with pure water droplets.
The theoretical and numerical study was conducted by Dr Saravanan Balusamy, Dr Sayak Banerjee from the Department of Mechanical and Aerospace Engineering and Prof Kirti Chandra Sahu from the Department of Chemical Engineering of IIT-H. Their work has been featured in a leading journal, International Communications in Heat and Mass Transfer.
Speaking about the findings of their study, Prof Kirti Chandra Sahu said, "While the lifetime of a small saliva droplet of the size one nanolitre is less than a minute, a normal size saliva droplet of 10 nanolitres takes more than 15 minutes to evaporate at room temperature and relative humidity of 50 percent. For high relative humidity, say of greater than 90 percent, the droplet stays on the surface for significantly longer, for about an hour. Of course, viruses could still be alive in dried droplets too, which requires further research involving biologists."
In the study, they found that the spread of the virus is minimal after drying droplets containing the virus. The study focuses on various factors affecting the drying time of saliva droplets whereas earlier studies were based on water droplets. Saliva droplets consist of salt, protein (mucin), and surfactant (
The angle made by a droplet on the surface - contact angle - plays important role in the drying time of the droplet and hence highly hydrophilic surfaces may be less susceptible to prolonged contamination. "A simple classical theoretical model developed in this study that takes into account the dynamic contact angle and the insoluble surfactants and is capable of accurately predicting the evaporation time under different conditions," said Dr Saravanan Balusamy
"The time taken by saliva droplets to dry also depends on the nature of the surface material on which it falls as it dictates how far the droplets have spread out," said Dr Sayak Banerjee.
The longest drying time was observed with the combination of low ambient temperature and high relative humidity, while the drying time progressively reduced as the humidity fell and the temperature rose. Researchers also found that for a fixed initial volume of the droplet, increasing the initial contact angle increases the drying time. This follows physical intuition, as a droplet with a smaller contact angle, has a greater wetting radius and the surface area for the same volume resulting in a larger interface area over which the diffusion-driven evaporation can occur. Thus, highly hydrophilic surfaces may be less susceptible to prolonged contamination in comparison to less hydrophilic surfaces, which is somewhat a counter-intuitive result.
The study conducted by the IIT-H faculty showed the importance of properly sanitising the contact prone surfaces in different seasons and in indoor areas that are air-conditioned. It suggested that people follow the World Health Origination (WHO) guidelines, such as wearing facemasks and maintaining social distance as preventive measures for transmission of COVID-19 through air.