Convalescent plasma therapy: Old wine in new bottle
By Newsmeter Network
Dr Ravi Andrews,
Do you remember that feeling on a rollercoaster? When the coaster suddenly drops down a slope, it feels like your stomach has been left at the top while the rest of your body comes to a jerk at the bottom. This is exactly how the medical fraternity felt when the ICMR Indian Council of Medical Research) ruled that plasma therapy was ineffective in the treatment of COVID-19 infection. The whole medical community was “banking” on Blood Banks as plasma therapy seemed to be the only hope for patients with life-threatening COVID-19 infections.
They were hoping it would be a game-changer in the fight against the virus. However, the ICMR burst this bubble with the results of their study on convalescent plasma therapy (CPT). Nevertheless, there is still hope that plasma therapy might work because the ICMR Study (labeled the PLACID trial) has been criticized by a lot of doctors. Before we look at the PLACID trial critically, let us understand more about convalescent plasma therapy.
The first reports of CPT date back to a century ago, in 1918, when it was used to treat the Spanish flu. Subsequently, it was used to treat varied infectious diseases like rabies, polio, and even measles. More recently, CPT has been used to treat Swine flu, Ebola, and MERS.
The process of CPT is simple. First, a patient who has recovered fully from COVID-19 is identified. Ideally, his antibody levels to the virus should be estimated (most people develop virus-neutralizing antibodies 2- 6 weeks after infection). After confirming that the neutralizing antibody levels are high enough, about 500 ml of blood is removed from the patient. This blood is sent to a machine called a centrifuge where the blood is separated into its three main components viz. Blood cells, Plasma and Platelets. The blood cells and the platelets are returned to the patient and the plasma (about 200 ml) is kept in the Blood Bank to use as CPT.
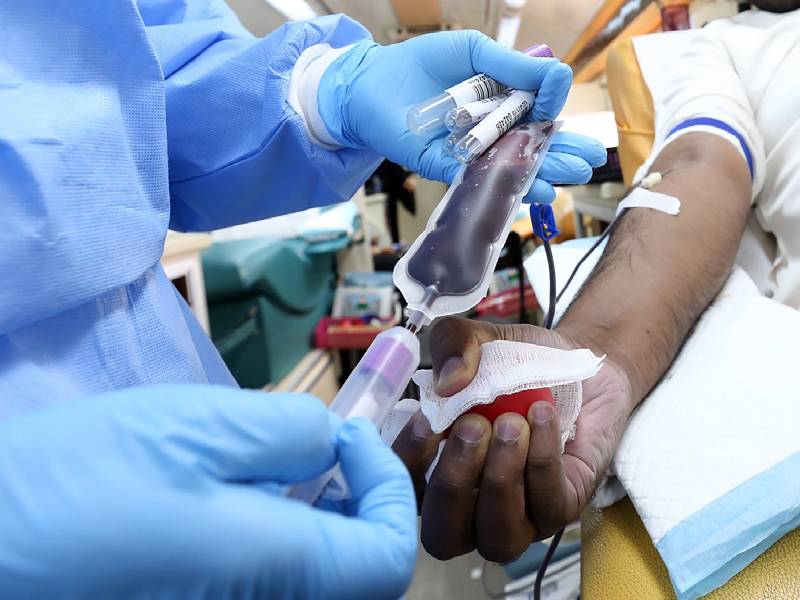
The donated plasma is transfused to infected patients. In most centers, two bags of convalescent plasma (200 ml each) are transfused. Each bag is transfused over two hours. The logic behind CPT is that it provides readymade antibodies to the patient to enable him to fight the infection even before he produces his own natural antibodies to the virus. Most patients produce their own antibodies after 7 -10 days. Obviously, the best time to use CPT is in the first 3- 5 days of infection. This kickstarts the immune response against the virus. Also, donated plasma is more useful if the donor has a high level of antibodies.
All this seems logically very sound but CPT also has certain risks which need to be considered –
- Transfusion reactions due to allergy.
- Transmission of viral infections like HIV, Hepatitis B, and C if the plasma is not tested properly.
- Lung injury sometimes occurs due to transfusions.
- Volume of plasma may be too much to handle for some patients and they may get breathless.
- Theoretically, COVID-19 virus may also be transmitted during transfusion of plasma.
These complications are very rare, less than 1% but it goes to show that CPT is not absolutely risk-free.
Considering the theoretical benefits of CPT, the minimal complications, and the lack of validated treatment at the time, the US Food and Drug Administration (FDA) authorized CPT for COVID- 19. From June 2020, the Union Health Ministry of India allowed CPT for sick patients as an ‘investigational therapy’. Many hospitals started using CPT for COVID-19 patients. Blood banks and social organizations started keeping lists of potential plasma donors and Whatsapp and other social media platforms had a field day forwarding these lists. Some unscrupulous people also began selling their plasma at exorbitant rates and other crooks began extorting money from victims of the COVID-19 virus.
The ICMR study (PLACID Trial) showed that CPT had no adverse reactions and did provide mild relief in symptoms. However, there was no reduction in the number of deaths due to COVID-19 infection. Hence CPT was not validated as a treatment by the ICMR. However, many doctors have disagreed with the results of the ICMR study pointing out technical flaws in it:
- The study was not peer-reviewed meaning it was not evaluated by a group of experts in the same field. Peer review is essential to showcase the quality of a study.
- In many donors, the level of neutralizing antibodies was not very high. In just 1/3 of donors were the antibody levels acceptably high.
- The number of patients was only 464, which is too small a number to reach a definitive conclusion.
Around the world, over 35,000 patients have been studied for the benefits of CPT in various studies. The conclusions from these studies are:
- CPT is more useful if it is used in the early stages – within 3 -5 days of the onset of symptoms.
- Its benefits are best if used in mild or moderately ill patients and not very sick, ventilated patients alone.
- The donor plasma should have high levels of neutralizing antibodies.
As it stands today, the jury is out on CPT as a mode of treatment for COVID-19. Currently, there are more than 74 high-quality studies ongoing around the world on the role of CPT in COVID- 19. Be patient, we will soon have an answer.
Dr Ravi Andrews is a Nephrologist at Apollo Hospital, Hyderabad.