DCA raids quack’s clinic in Bhadradri-Kothagudem, seizes drugs with misleading claims
Officials found 25 varieties of medicines, including antibiotics and analgesics, stocked without a valid drug license
By Anoushka Caroline Williams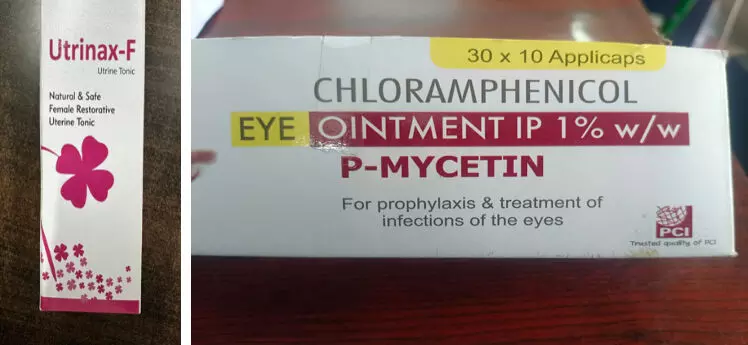
DCA raids quack’s clinic in Bhadradri-Kothagudem, seizes drugs with misleading claims
Hyderabad: The Telangana Drugs Control Administration (DCA) has intensified efforts to curb illegal medical practices and misleading drug advertisements. Recent raids in Bhadradri-Kothagudem and other districts resulted in the seizure of drugs worth thousands of rupees and actions against unlicensed practitioners and misleadingly labelled medicines.
Raid on quack’s clinic in Bhadradri-Kothagudem
On November 27, the DCA raided the clinic of Jangam Venkateshwarlu, an unqualified practitioner operating in Bommanapally Village, Tekulapally Mandal, Bhadradri-Kothagudem District.
Officials found 25 varieties of medicines, including antibiotics and analgesics, stocked without a valid drug license. Among the seized items, 12 varieties were labelled as ‘physician’s samples.’ The estimated value of the stock was Rs 25,000.
“Indiscriminate sale of antibiotics by unqualified persons can lead to public health crises, such as antimicrobial resistance,” said Ch. Sampath Kumar, drugs inspector, Bhadradri-Kothagudem, who led the operation.
Samples from the seized stock have been sent for analysis. Further investigations are ongoing, and legal action will be initiated against the offenders, including wholesalers and dealers supplying medicines without verifying the recipient’s license.
Misleading drug advertisements targeted
In separate actions, the DCA seized medicines with misleading claims regarding their therapeutic uses. These products, detected during raids across various districts, violated the Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954, which prohibits advertisements for treating certain diseases and disorders.
Seized medicines and misleading claims
The medicines with misleading claims and their manufacturing information were:
P-Mycetin eye ointment for ‘prophylaxis and treatment of eye infections’, manufactured at Eastern Health Care, Uttarakhand.
Utrinax-F tonic treats ‘disorders of menstrual flow’, manufactured at Areva Herbals, Punjab.
ED Phenicol eye ointment for ‘prophylaxis and treatment of eye infections’, manufactured at Eamon Drugs in Madhya Pradesh.
Nihri syrup treats ‘kidney stones and prostate disorders’, and is manufactured at Makin Laboratories in Madhya Pradesh.
Legal consequences
Violations of the Drugs and Cosmetics Act, as well as the Drugs and Magic Remedies (Objectionable Advertisements) Act, can lead to imprisonment of up to five years. Stringent actions are planned against wholesalers, dealers, and manufacturers involved in such practices.
Public awareness and reporting
The DCA urged the public to report illegal activities concerning medicines, including unauthorised manufacturing or misleading advertisements. Complaints can be made through the DCA’s toll-free number, 1800-599-6969, operational from 10:30 am to 5 pm on working days.
“Ensuring the safety and efficacy of medicines is a collective responsibility. Public participation in reporting violations will help us maintain high drug safety standards,” said VB Kamalasan Reddy, IPS, director general of the Drugs Control Administration, Telangana.