5 common myths about CAR T-Cell therapy busted
Dr Rakesh Reddy Boya breaks down the myths behind CAR T-cell therapy and puts forward the facts about how the process really works.
By - Dr Rakesh Reddy Boya |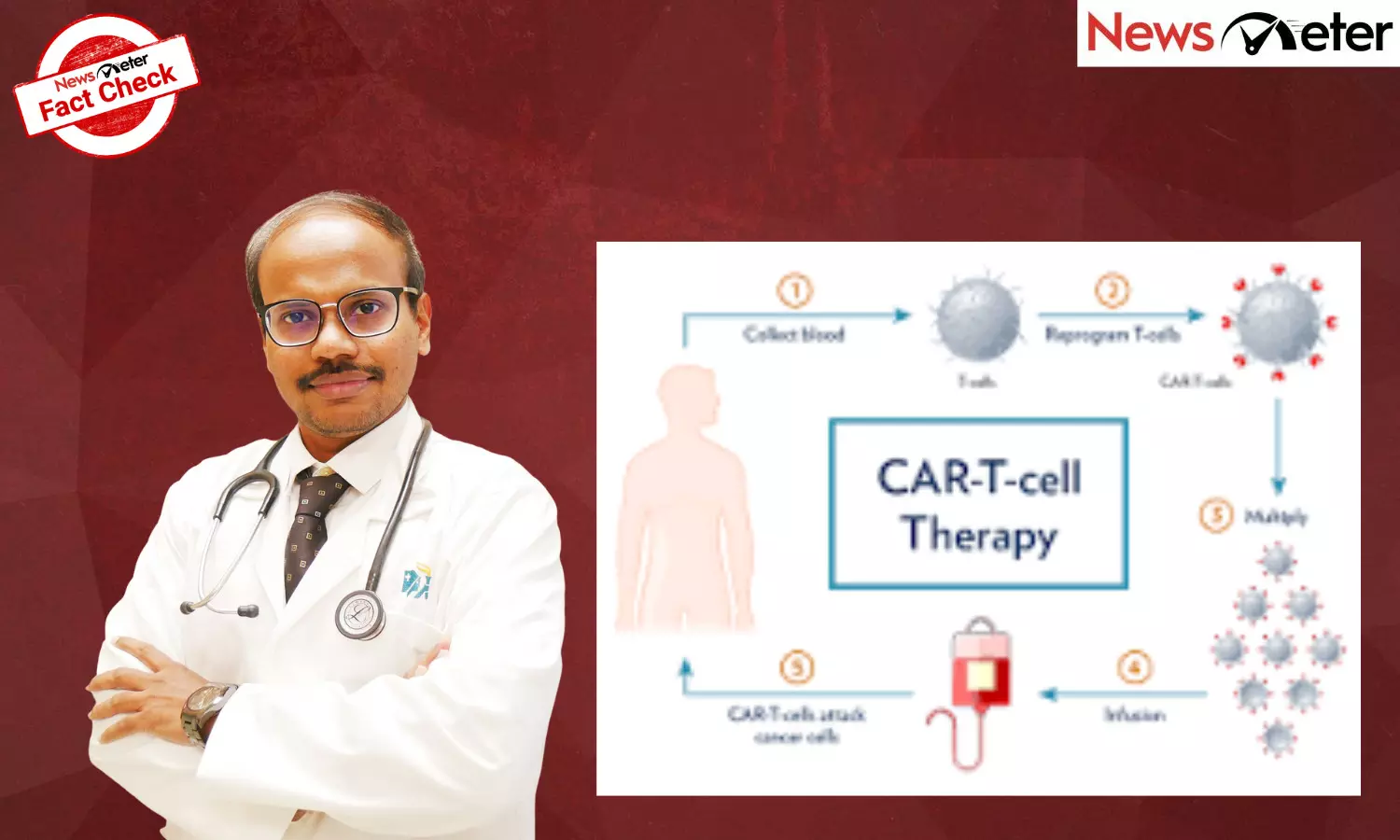
Hyderabad: Immunotherapy has transformed the way several cancers are treated, and among its most advanced forms is CAR T-cell therapy, a treatment that uses a patient’s own immune cells to find and destroy cancer cells.
In India, the field has seen remarkable progress in recent years. The Indian Council of Medical Research (ICMR) notes that multiple clinical trials for CAR T-cell therapy are currently underway across major institutes, including Tata Memorial Centre, IIT Bombay, and AIIMS Delhi. The country’s first indigenously developed CAR T-cell product received regulatory approval in 2024, marking a turning point for accessible immunotherapy.
Yet, misconceptions remain. Patients often approach oncologists with mixed feelings of hope and uncertainty, influenced by incomplete information online. Clarifying these myths helps patients and families make informed decisions rooted in science rather than speculation.
Dr Rakesh Reddy Boya breaks down the myths behind CAR T-cell therapy and puts forward the facts about how the process really works.
Myth 1: CAR T-cell therapy is a last resort when all other treatments fail
While it is true that CAR T-cell therapy was initially offered to patients with cancers that had returned after multiple treatments, this landscape is changing rapidly. Global data and early Indian trials show that earlier use in specific cases may improve survival and long-term remission rates.
For example, studies published in the New England Journal of Medicine demonstrated that patients with certain relapsed lymphomas achieved remission rates of 60–70 per cent after CAR T-cell therapy.
In India, oncologists at Tata Memorial Hospital are exploring its application in younger patients and those with aggressive blood cancers where conventional chemotherapy provides limited benefit.
CAR T-cell therapy is not only a ‘last resort.’ It represents a specialised option best suited to particular cancer types and stages, determined after genetic and molecular evaluation.
Myth 2: CAR T-cell therapy can treat all cancers
Currently, CAR T-cell therapy is most effective for blood cancers such as acute lymphoblastic leukaemia (ALL), certain types of non-Hodgkin’s lymphoma, and multiple myeloma. Solid tumours, such as those of the lung, breast or colon, pose additional challenges because cancer cells in these organs hide behind dense tissue barriers and complex microenvironments that limit immune access.
Research teams at AIIMS Delhi and IIT Bombay are developing newer generations of CAR T-cells designed to overcome these barriers. While the science is promising, widespread use for solid tumours is still several years away. Understanding these distinctions helps patients maintain realistic expectations and avoid misinformation.
Myth 3: CAR T-cell therapy works instantly
Unlike chemotherapy, which begins acting as soon as the drugs circulate in the bloodstream, CAR T-cell therapy follows a precise and time-intensive process.
First, the patient’s T-cells are collected through a blood-filtration procedure called leukapheresis. These cells are then genetically modified in a laboratory to express a “chimeric antigen receptor” (CAR) that recognises cancer cells. After several weeks of preparation, the modified cells are infused back into the patient.
Clinical response is monitored over the following weeks or months. Tumour reduction may not be immediate, as the modified T-cells expand and continue to fight cancer long after infusion. This gradual mechanism explains why ongoing observation is essential, even when patients initially feel well.
Myth 4: Side effects are unbearable and uncontrollable
Like any advanced therapy, CAR T-cell treatment carries potential risks, but they are predictable and manageable with current medical protocols. The most notable side effect, cytokine release syndrome (CRS), occurs when activated T-cells release immune chemicals into the bloodstream, leading to fever or low blood pressure.
In clinical settings, doctors closely monitor patients for early signs of CRS and treat it effectively with targeted drugs such as tocilizumab. The Indian Journal of Medical and Paediatric Oncology reported that in controlled environments, over 90 per cent of patients experiencing CRS recover fully within days.
Neurological effects such as confusion or tremor can also occur, but are usually reversible. Experienced cancer centres now have dedicated teams trained in recognising and treating these complications early, making the procedure considerably safer than in its early years.
Myth 5: CAR T-cell therapy is inaccessible or unaffordable in India
Until recently, cost and availability were major barriers because therapies had to be imported from the United States or Europe. The introduction of India’s first home-grown CAR T-cell product, NexCAR19, developed jointly by IIT Bombay and Tata Memorial Centre and approved by the Central Drugs Standard Control Organisation (CDSCO) in 2024, has changed this equation.
Indigenous manufacturing has reduced cost significantly — from several crore rupees for imported products to an estimated Rs 30–40 lakh for local therapies. Several government and private hospitals in Mumbai, Delhi, and Bengaluru have established dedicated facilities for CAR T-cell preparation and patient care, with expansion planned through public-private partnerships under the National Biopharma Mission. This progress represents an important step toward equitable access to precision immunotherapy in India.
CAR T-cell therapy symbolises a new era in cancer care, one where treatment is personal, targeted, and powered by the patient’s own biology. Ongoing trials in India aim to extend its benefits to more cancer types, shorten manufacturing time, and reduce costs further.
For patients, the takeaway is clear: CAR T-cell therapy is neither a miracle cure nor an experimental gamble. It is a scientifically validated option that offers meaningful hope for certain cancers when guided by an experienced oncology team. Staying informed, asking questions, and seeking treatment in accredited centres ensures both safety and the best possible outcomes.
(Dr Rakesh Reddy Boya is a Senior Consultant of Medical and Hemato Oncology and BMT Physician at Apollo Cancer Centres in Visakhapatnam)