Fact Check: NimOset-P and Nice tablets are banned? No, here are the facts
Social media users are claiming that NimOset-P and Nice tablets used as painkillers have been banned by the Indian government.
By - Newsmeter Network |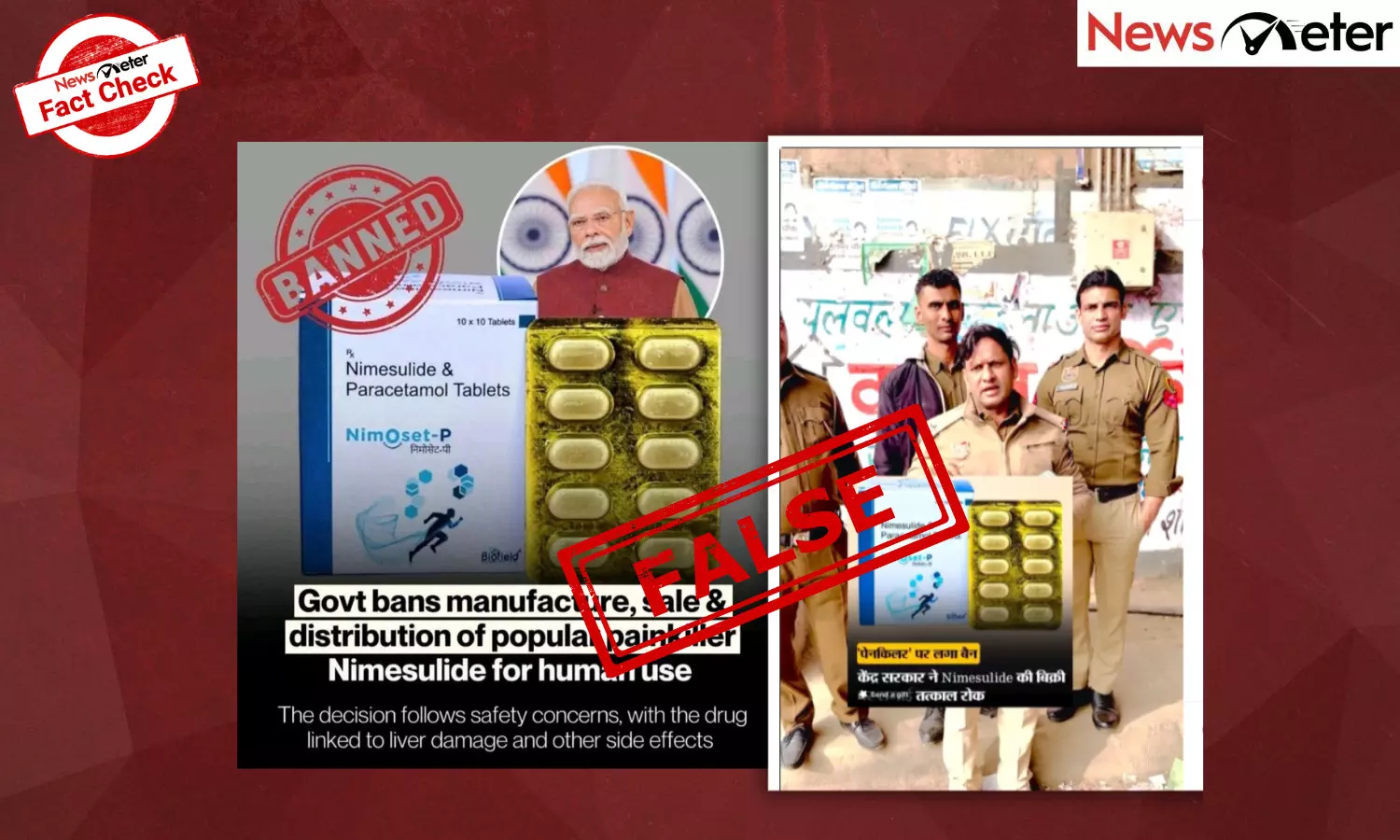
Claim:The Indian government has banned the manufacture and sale of NimOset-P and Nice tablets after they were found to have harmful side effects
Fact:The claim is false. The government has banned only Nimesulide formulations containing more than 100 mg, not the commonly available 100 mg tablets like NimOset-P and Nise.
Hyderabad: A video circulating on Instagram, shared by user ‘radhikatimeshisar’, shows a person dressed in a police uniform warning people against consuming a medicine called NimOset-P tablet. The thumbnail of the video has the text, “This medicine can take your life. Watch which one.” It further states, “Ban imposed on a painkiller. The Central Government has immediately stopped the sale of nimesulide.”
The caption reads, “Paracetamol combined with other drugs due to safety concerns, such as those with cetirizine or nimesulide, has been banned.”
In the video, the person wearing a police uniform says, “The medicine you are seeing has been immediately banned by the government. This medicine was earlier prescribed for headaches and mild fever. First, it was banned for children below 12 years. Then it was banned for animals. Now, from December 29, 2025, this medicine has been completely banned for everyone with immediate effect. If this medicine is found at any medical store, it is illegal. Do not consume this medicine at all. It is causing the heart rate to stop and leading to several heart-related diseases.” (Translated from Hindi)
Similarly, Dr Akansha Sharma, who identifies as a Gut and Hormone Health Specialist, posted on Instagram and LinkedIn with a photograph of NimOset-P marked as banned. The post was later edited, but the photograph remained.
Another post about ‘Nise’ tablets labelled as ‘Nice’ tablets by Dr Reddy’s Laboratories was shared with similar banned claims.
Multiple posts showing NimOset-P tablet with banned stamps along with Prime Minister Narendra Modi’s image have been shared across platforms.
Fact Check
NewsMeter found that the viral claim is false. The government has banned only Nimesulide formulations containing more than 100 mg, not the commonly available 100 mg tablets like NimOset-P and Nise.
What the government actually banned
According to the Ministry of Health and Family Welfare’s Gazette Notification, dated December 29, 2025, “Whereas the Central Government is satisfied that the use of all oral formulations containing Nimesulide above 100 mg in immediate release dosage form are likely to involve risk to human beings and that safer alternatives to the said drug is available; Now, therefore, in exercise of the powers conferred by section 26A of the Drugs and Cosmetics Act, 1940, and after consultation with the Drugs Technical Advisory Board, the Central Government, hereby prohibits the manufacture, sale and distribution of the following drug, with immediate effect, namely: All oral formulations containing Nimesulide above 100 mg in immediate release dosage form.”
The government has banned Nimesulide containing more than 100 mg, not formulations with exactly 100 mg or less.
What are the products in the claim?
Two drugs shown in viral posts—NimOset-P tablet and Nise tablet—contain only 100 mg of Nimesulide, not more than 100 mg. The website of NimOset-P tablet manufacturer Biofield Pharma describes the product as ‘Nimesulide 100 mg + paracetamol 325mg is an effective combination of two medications under the brand name ‘NIMOSET-P’ available in tablet form.”
Similarly, Nise tablets manufactured by Dr Reddy’s Laboratories came out with a clarification on LinkedIn under one of the posts: “Hi Akram Ahmad, PhD, we would like to clarify that the recent government directive pertains to Nimesulide doses above 100 mg. Our company does not manufacture or distribute Nimesulide in doses above 100 mg.”
Dr Reddy’s Laboratories also noted: “Additionally, the product pack shot used in your post does not accurately represent the actual product, as it contains an incorrect logo and product name. As you are aware, the logo and product name constitute the company’s intellectual property, and any alteration is a sensitive matter that may cause confusion among patients and the public.”
The post by Akram Ahmad was later edited and a clarification was added, but the misleading photograph was not removed from LinkedIn. Most posts have been edited in captions, but the photographs showing banned stamps remain.
What should the public know about painkillers
Hyderabad-based neurologist Dr Sudhir Kumar explained what people need to understand about Nimesulide: “The Government of India has banned all immediate-release oral formulations of Nimesulide containing more than 100 mg, citing safety concerns. Nimesulide is a pain-reliever (NSAID). While effective, it is associated with dose- and duration-related adverse effects, especially on the liver.”
Dr Kumar outlined possible adverse effects of Nimesulide, noting that risk increases with higher doses and longer use: “Liver injury (hepatitis, liver failure; rare but serious), nausea, vomiting, abdominal pain, gastritis, GI bleeding, kidney dysfunction (especially in dehydration or elderly), and increased cardiovascular risk (like other NSAIDs). Liver toxicity has been reported even with short-term use in some individuals, and risk rises with doses greater than 100 mg per day.”
He specified who may take Nimesulide if prescribed: “Adults, at the lowest effective dose, for the shortest possible duration (usually five days or less), and only under medical advice.”
Dr Kumar also listed who should avoid Nimesulide: “Children and adolescents, anyone with liver disease, heavy alcohol users, elderly with kidney disease, people taking other liver-toxic drugs, and long-term pain management patients.”
He recommended safer alternatives for pain and fever: “Paracetamol (first choice for most), ibuprofen or naproxen (with caution, food and short duration), and non-drug measures: rest, ice or heat, physiotherapy.”
Stronger doesn’t mean safer
Dr Kumar emphasised important principles: “‘Stronger’ does not mean safer. Painkillers are not harmless, especially when overused. Avoid self-medication and high-dose combinations. Always use the lowest dose for the shortest time. Public awareness can prevent drug-related harm.”
The viral claims that NimOset-P and Nise tablets have been completely banned by the Indian government are false. On December 29, 2025, a government notification banned only Nimesulide formulations containing more than 100 mg in immediate release dosage form.
Both NimOset-P and Nise tablets contain exactly 100 mg of Nimesulide, which falls within the permitted limit. These products remain legal and available with a proper medical prescription. The manufacturer, Dr Reddy’s Laboratories, has officially clarified this misinformation.
While Nimesulide does carry safety concerns related to liver toxicity—which is why higher doses are banned—the commonly prescribed 100 mg formulation remains approved for use under medical supervision. The claim is false.