Has WHO disapproved Bharat Biotech's `Covaxin'?
Some netizens have shared a message claiming that `COVAXIN’ has not been approved by the World Health Organization (WHO).
By Shimron Diana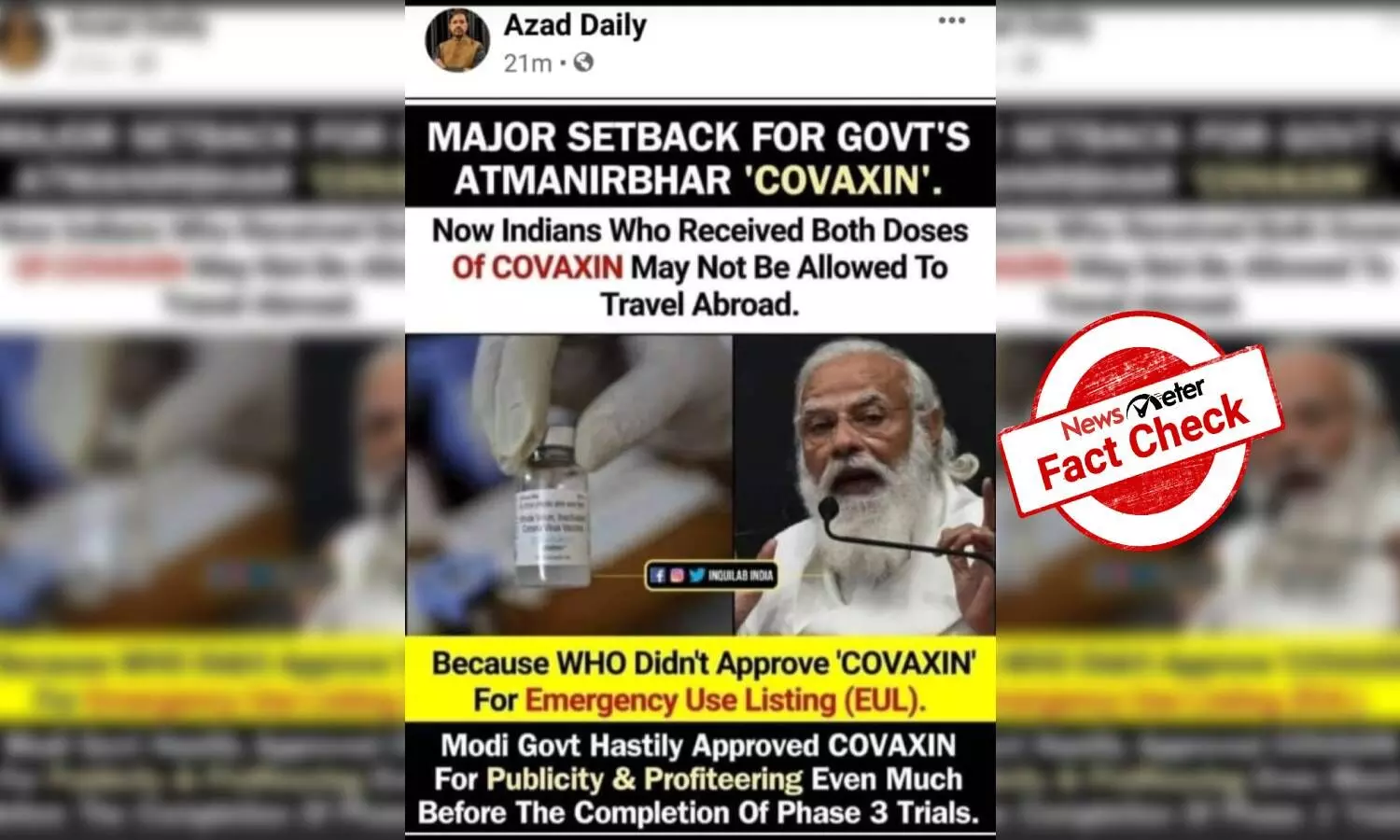
Hyderabad: Some netizens have shared a message claiming that `COVAXIN' has not been approved by the World Health Organization (WHO).
"Major setback for government's Atmanirbhar "COVAXIN". Now Indians who received both doses of COVAXIN may not be able to travel abroad, because WHO didn't approve 'COVAXIN' for Emergency Use Listing (EUL). Modi Government hastily approved COVAXIN for publicity and profiteering even much before the completion of phase 3 trials (sic)," reads the post.
This has given anxiety to many social media users.
Fact Check
The claim is partially true. COVAXIN has been administered to a lot of people even before publishing the third stage of clinical trial results. It is in the process of being approved by the WHO. Later Bharat Biotech said its vaccine efficacy is 81 percent.
NewsMeter first tried to find if the WHO has disapproved of COVAXIN. "Bharat Biotech has submitted its 'expression of interest' to be certified, but added that "more information" is required," said the WHO.
The pre-submission meeting will be planned for May or June.
A tweet by Bharat Biotech said that application for EUL has been submitted to the WHO and regulatory approvals are expected by July – September 2021.
— BharatBiotech (@BharatBiotech) May 25, 2021
According to The Times of India report, the Indian firm must now submit a dossier. If it is accepted, the WHO will issue a statement of assessment before it decides to include COVAXIN in the EUL.
Hence it is false that COVAXIN has not been approved by the WHO. It is still in the stages of approval.
Last week 27 countries in the European Union agreed to ease travel restrictions on non-EU visitors who are fully vaccinated. The WHO has come up with an 'emergency use list' which holds names of the vaccines that people should administer in order to travel. COVAXIN was not a part of the list, and this caused concern among people who had taken its two doses.
This virally circulated message is misleading people by claiming that the COVAXIN was not approved but the fact is that it is still in the stages of approval. The government of India is aware of people's concerns and is eager to ensure that COVAXIN receives clearance at the earliest.
The viral claim is true to the extent that India's drug regulator gave emergency approval for a locally produced Coronavirus vaccine, `COVAXIN', before the completion of its trials. The vaccine was approved for restricted use in an emergency situation in the public interest as an abundant precaution, in a clinical trial mode, especially in the context of infection by mutant strains.
Despite being in the third phase of clinical trials, COVAXIN has been administered to thousands of people. It is not known if the government has done this for publicity or for profiteering. An all-India drug action network had said that it was baffled about the scientific logic behind approving an incompletely studied vaccine. The vaccine was later approved and is found to be safe.
Here is a report on the approval of an incompletely studied vaccine COVAXIN by BBC.
Hence, the virally circulated claim is partially true. It is true in that COVAXIN was administered to people even before the third stage of clinical trials results was published but false in its inference that the WHO has disapproved COVAXIN. The vaccine is still in the stages of being approved.