Hyd firm Bharat Biotech's intranasal Covid-19 vaccine completes trials
The data from phase III human clinical trials are submitted for approval to the National Regulatory Authorities
By Newsmeter Network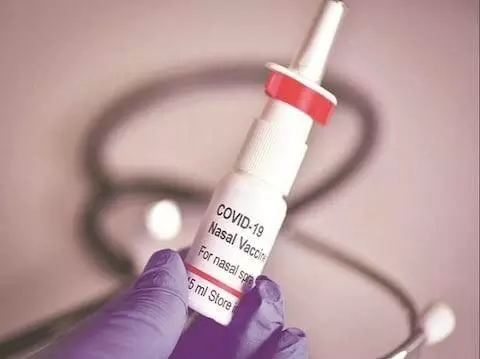
Hydrerabad: BBV154 intranasal vaccine for Covid-19, developed by Hyderabad-based Bharat Biotech and partly funded by the Central government, has proven to be safe, well-tolerated, and immunogenic in controlled clinical trials. Two separate and simultaneous clinical trials were conducted to evaluate BBV154. A primary dose of two-dose schedule and a heterologous booster dose were studied.
Primary dose trials were conducted on 3,100 subjects and the trials were carried out in 14 sites across India. Heterologous booster dose trials were conducted on 875 subjects and these trials were conducted in nine sites across India. The data from phase III human clinical trials are submitted for approval to the National Regulatory Authorities.
The immunogenicity of the vaccine has elicited long-term memory T and B cell responses against the different omicron variants.
Suchitra K. Ella, the joint managing director of Bharat Biotech, said, "On this 75th Independence Day, we are proud to announce the successful completion of clinical trials for the BBV154 intranasal vaccine. We stay committed and focused on innovation and product development. If approved, this intranasal vaccine will make it easier to deploy in mass immunization campaigns with an easy-to-administer formulation and delivery device."
BBV154 has the double benefit of enabling faster development of variant-specific vaccines and easy nasal delivery. This will help in mass immunisation programmes. BBV154 is stable at 2-8°C for easy storage and distribution. Bharat Biotech has established large manufacturing capabilities at multiple sites across India, including Gujarat, Karnataka, Maharashtra, and Telangana, with operations across India.