Hydroxyurea gets DCGI approval for sickle cell anaemia treatment
The approval currently legalizes the drug to be used at standard doses for treatment of SCA. It also sets up a stage for designing various formulations of smaller dose sizes.
By Newsmeter Network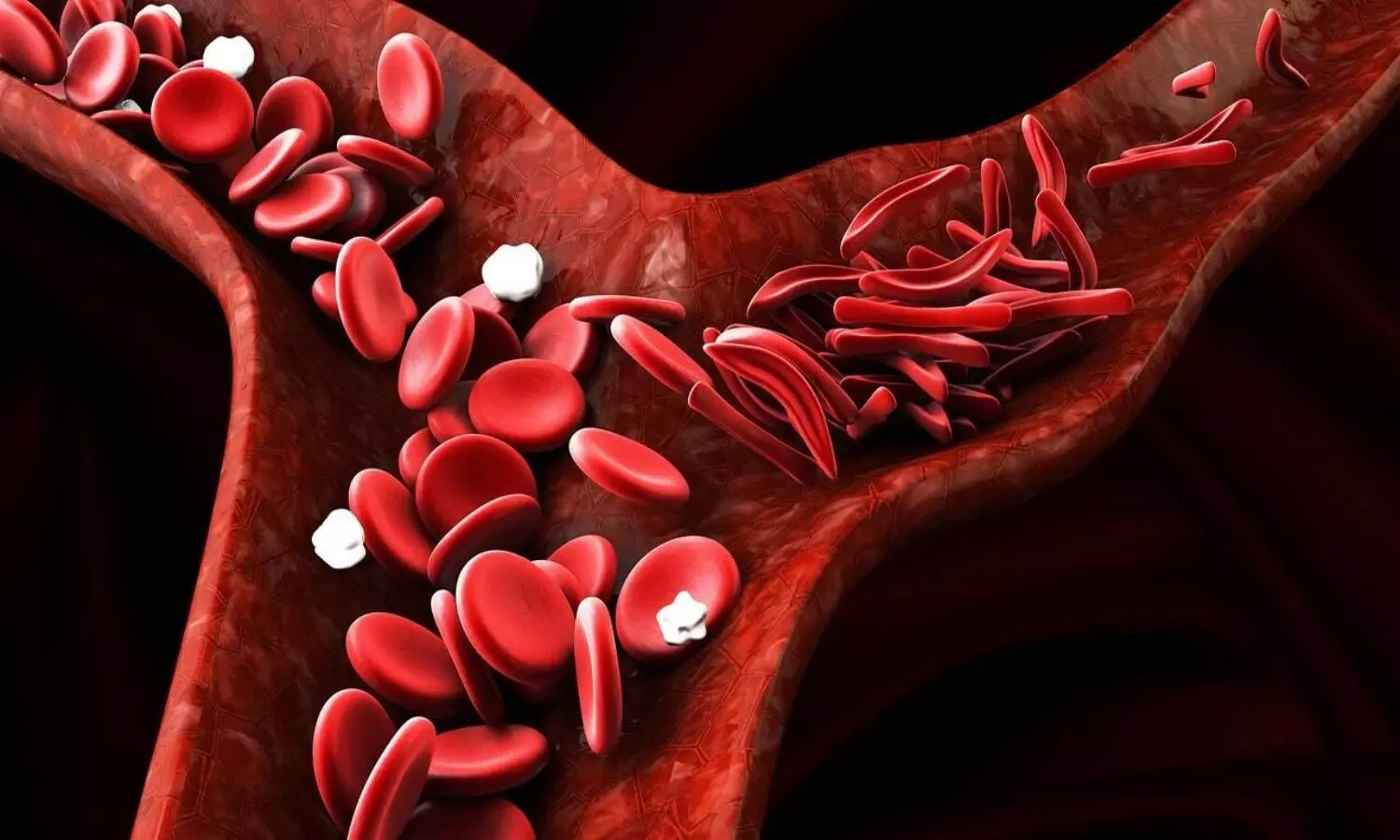
Hyderabad: A committee of experts constituted by the Central Drug Standard Control Organization (CDSCO) has approved the marketing of hydroxyurea for the treatment of sickle cell anaemia (SCA).
The approval currently legalizes the drug to be used at standard doses for treatment of SCA. It also sets up a stage for designing various formulations of smaller dose sizes that promise higher compliance rates in children suffering from sickle cell anaemia and may even lead to syrup-based formulations.
"This is a landmark achievement and adds to the advantages of identifying patients through a targeted screening programme. While one of the major focuses of the screening programme is to avoid birth of affected children through genetic and social counselling, this approval provides comprehensive treatment to the identified patients. The message now needs to reach clinicians across the country so that they can use Hydroxyurea regularly for their patients," said Dr. Giriraj R. Chandak, chief scientist at CSIR-CCMB and mission director leading the CSIR-Sickle Cell Anaemia (CSIR-SCA) Mission.
Under CSIR-SCA Mission with six CSIR labs and three government hospitals in Chhattisgarh, Madhya Pradesh, and Maharashtra, scientists and clinicians are trying to address various lacunae in SCA diagnosis and disease management. The focus is on identifying patients through population-based screening in states with high disease prevalence and helping the family with proper treatment and preventing the disease in the next generation. One of the major objectives of the mission has been obtaining approval for use of hydroxyurea for the treatment of SCA.
What is SCA?
SCA is a common genetic disorder among Indians affecting red blood cells. It is transmitted by parents who carry a defective beta-globin gene without themselves suffering. Like most genetic disorders, SCA has no cure but has symptomatic treatments for pain, anaemia, and Vaso-occlusive crisis. One of the rather inexpensive drugs, hydroxyurea, largely used as an anti-cancer agent, was till now used in SCA treatment without any formal approval. The commercially available hydroxyurea formulations are made with its anti-cancer role in mind and hence are of big quantity size (minimum 500 mg).
SCA children typically weigh less and consequently, their dosage size needs to be much smaller. Given the fixed and larger size of currently commercially available hydroxyurea capsules, it is difficult to give the correct doses to SCA patients. With DCGI approval, it also helps to design various formulations of smaller dose sizes that promise higher compliance rates in children suffering from SCA and may even lead to syrup-based formulations.
Close to 0.4% of the population suffers from the disease while 10% of people are carriers who give birth to new SCA patients. The disease is well-known in tribal populations and is prevalent in the general populations in states like Maharashtra, Madhya Pradesh, Chhattisgarh, and Odisha. The disease starts early in life and the affected children have persistent pain, low amount of haemoglobin (anaemia), low energy, reduced growth plus other abnormalities and multiple episodes of frequent severe pain better known as Vaso-occlusive crisis.