All about Hyderabad based Bharat Biotech's nasal vaccine trials
By Sumit Jha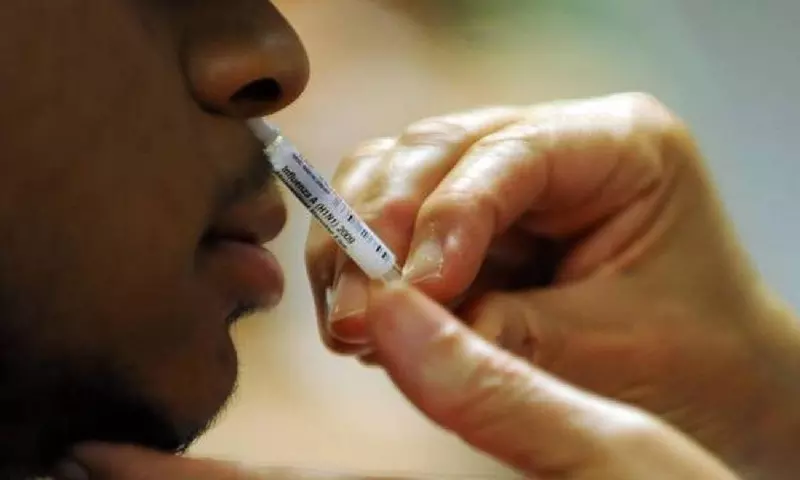
Hyderabad: Bharat Biotech has started Phase 1 clinical trial for its nasal Covid-19 vaccine `BBV154' in Hyderabad. A Hyderabad hospital, one of the four facilities, shortlisted across the nation for Phase 1 of the clinical trial gave the vaccine to two of the 10 shortlisted volunteers on Friday.
Both the patients who have received doses are healthy and doing fine, sources said. According to the Central Trail Registry of India (CTRI), these trials will be conducted on 175 participants at health centers in Patna, Chennai, Nagpur, and Hyderabad.
Bharat Biotech was given the permission to conduct the first phase of the clinical trial for its intranasal Covid-19 vaccine BBV154 by the Drugs Controller General of India (DCGI) on March 2. On January 8, Bharat Biotech approached the Drugs Controller General of India (DGCI) and sought permission to conduct Phase 1 and Phase 2 clinical trials of the intranasal vaccine. The subject expert committee deliberated on the application and recommended granting permission for Phase 1 trials on Tuesday.
Bharat Biotech's BBV154 is the first published attempt at getting an intranasal vaccine against coronavirus. The company has tied up with the Washington University School of Medicine in St Louis to develop an intranasal vaccine for COVID-19. Bharat Biotech is developing the nasal vaccine in an international collaboration with virologists of the University of Wisconsin, Madison, and private company `FluGen'.
"An intranasal vaccine stimulates a broad immune response neutralizing IgG, mucosal IgA, and T cell responses and creates an immune response at the site of infection (in the nasal mucosa) essential for blocking both infection and transmission of Covid-19." reads the Bharat Biotech website.
It also adds that the nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa. The administration of this is non-invasive and Needle-free which does not require trained health care workers.
Until now, all the vaccines against coronavirus across the world are intramuscular.