Paediatric vaccine for pneumonia by Hyd-based Biological E Ltd gets nod
By Kaniza Garari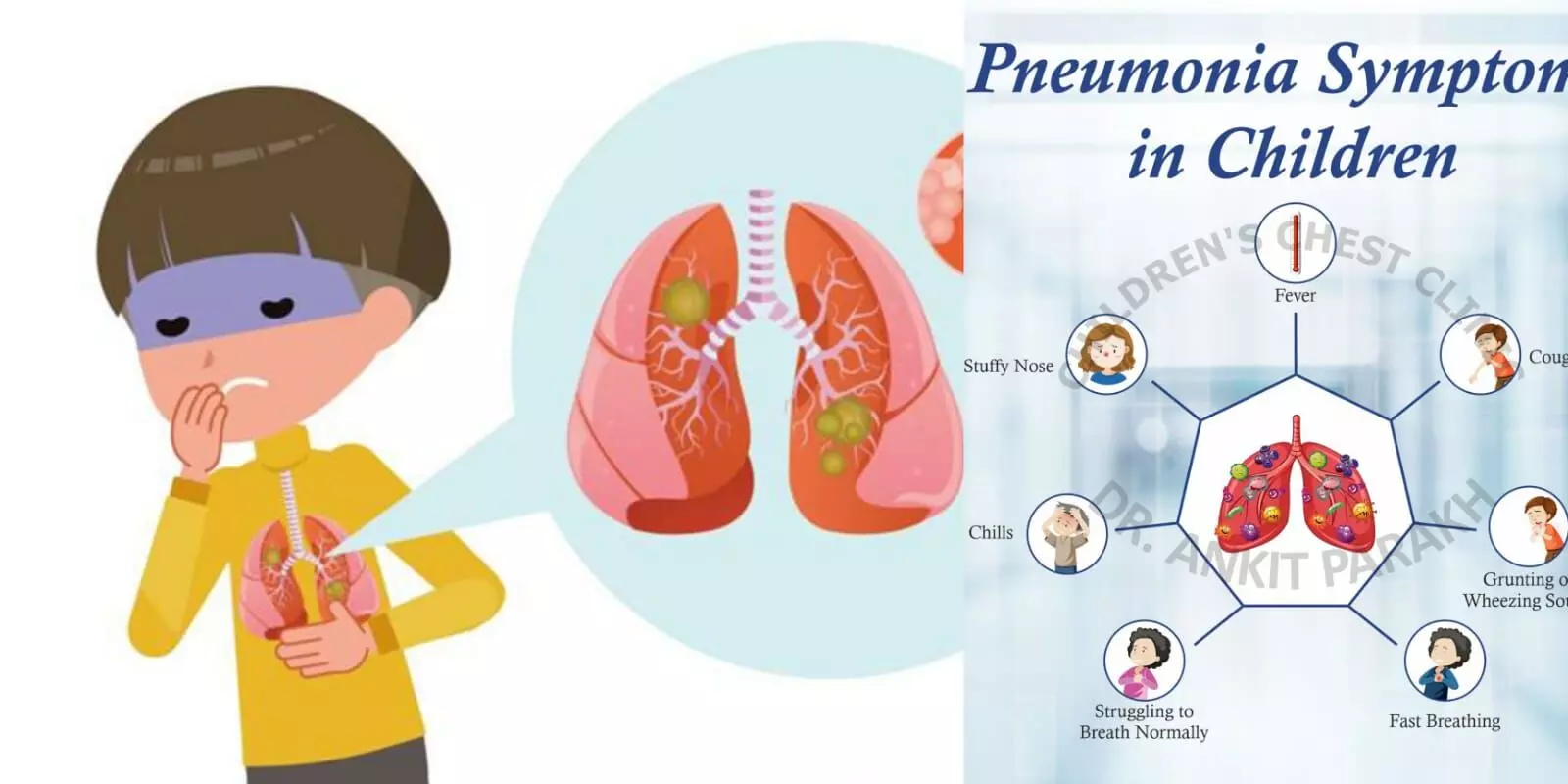
Hyderabad: Paediatric vaccine for pneumonia of Biological E. Limited has been approved by the Subject Expert Committee of Central Drugs Standard Control Organization. The 14-valent paediatric vaccine called PCV14 will be available in single and multi-dose. The PCV 14 can be administered to infants at 6, 10 and 14 weeks of age.
Pneumonia infection is a leading cause of mortality in India in children below 5 years of age. Prevalence of pneumonia in children younger than 5 years in India is 675 cases per 1000 cases in the year 2000. Fatality rate is 5 to 10 percent in hospitalized patients.
The vaccine contains 14 serotypes which will be able to tackle the growing infections in children and improve the response of the immunity. One month after 3rd dose of vaccination, there is substantial increase in immune response according to the results of the clinical trials.
The safety analysis revealed that all the adverse events were mild to moderate. Ms. Mahima Datla, Managing Director, Biological E. Limited, said "We are delighted with this remarkable development. BE's PCV14 will protect millions of infants worldwide and contribute to the prevention of invasive pneumococcal disease. We are working with WHO and other global regulatory agencies to make this vaccine available globally. "